For centuries, humanity marked time chronologically. Today, that measure is rapidly being challenged, particularly when it comes to longevity.
By John Heilprin
Dec. 4, 2025
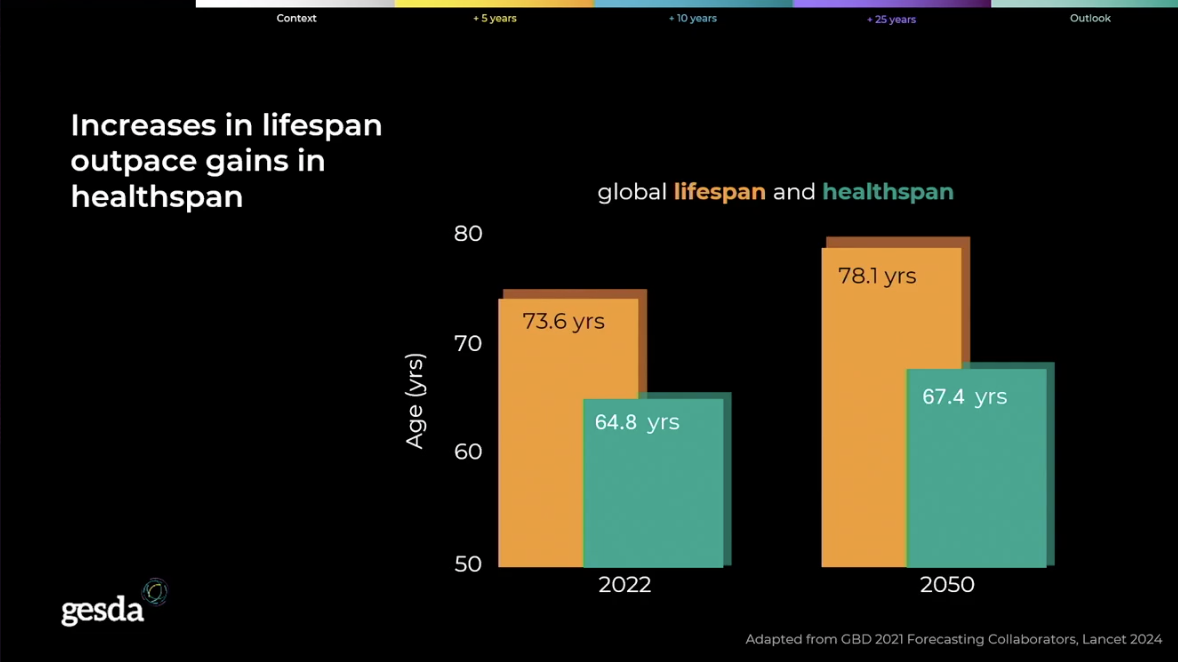
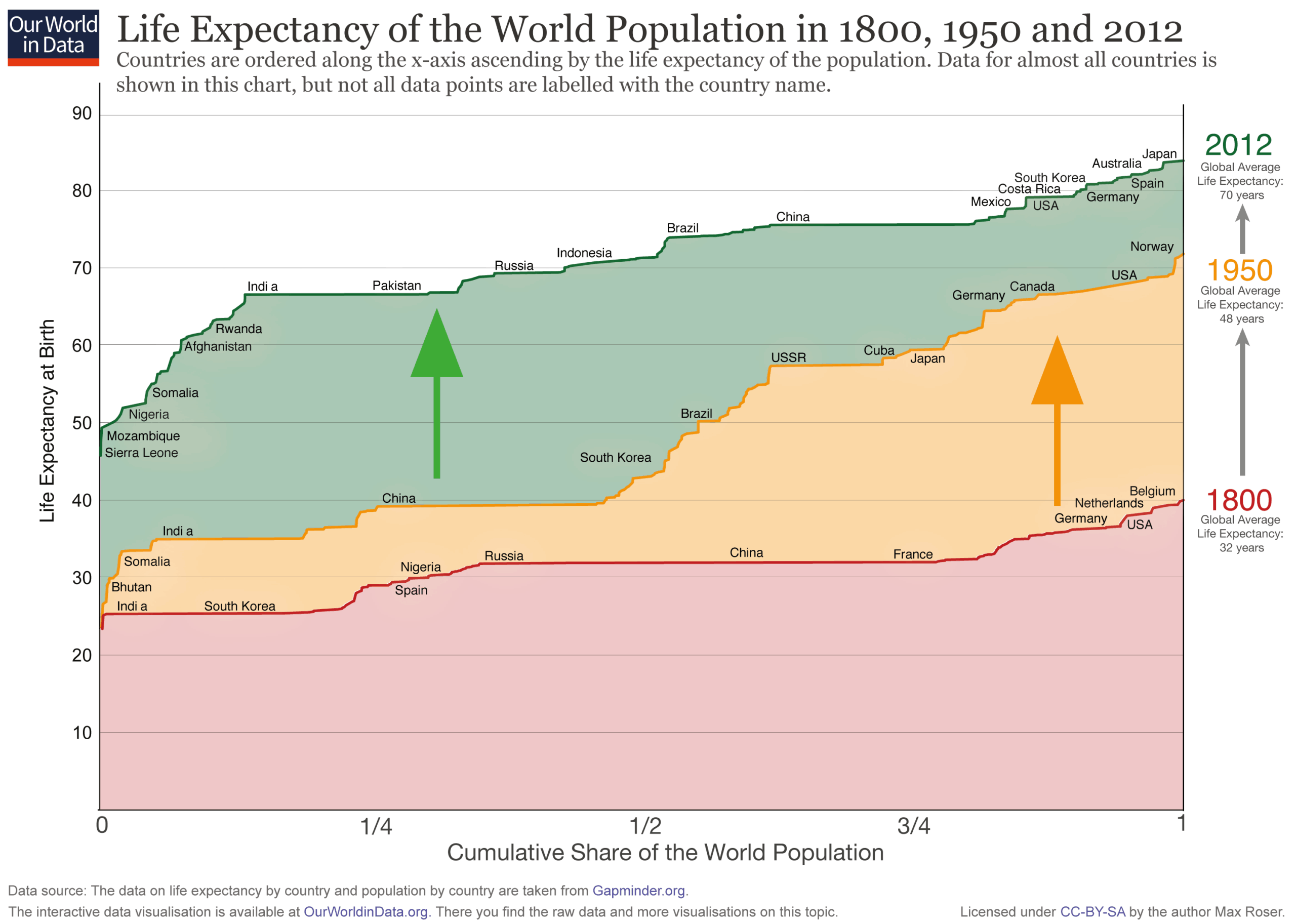
The dramatic increase in global lifespan over the past two centuries is one of humanity’s greatest achievements. The average global life expectancy stood at just 32 years in 1900, a figure that more than doubled to 73 years in 2023.
The measure, known as period life expectancy, is the average number of years a hypothetical newborn would live if exposed to the current year’s age-specific death rates. This rise was achieved not only through a sharp decline in child mortality, but also by delaying death at all ages through improvements in sanitation, nutrition, and medicine.
Now, the shift in focus from lifespan to healthspan, which scientists view as the portion of a person’s life that is lived in optimal health, is no longer considered a theoretical pursuit. Driven largely by emerging breakthroughs in molecular diagnostics and targeted drug development, longevity science is accelerating toward clinical reality, making the opportunity for radical health extension a central theme in the 2026 GESDA Science Breakthrough Radar®.
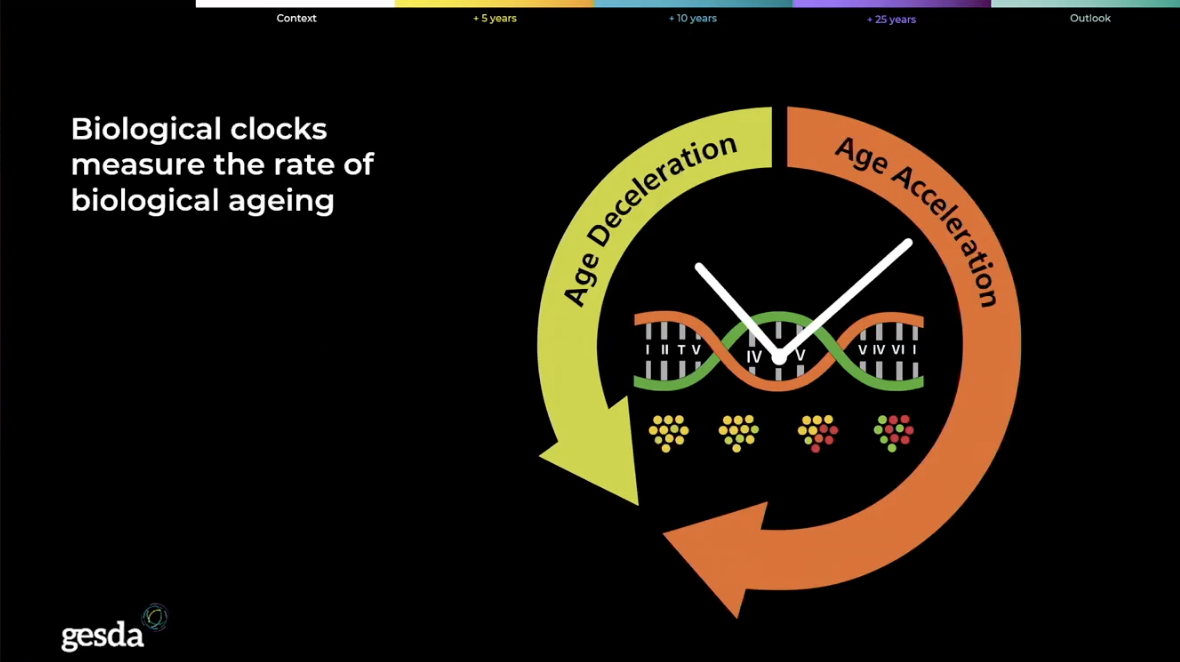
A growing focus is on biomarker‑derived measures of physiological aging, as a way to diagnose accelerated aging and target interventions. The Radar emphasizes that validating biomarkers is central to using biological age to test interventions, because biomarkers are statistical tools that improve with larger, well‑benchmarked population datasets. The acceleration, however, creates a global governance crisis that demands diplomatic focus, as detailed in the Radar.
The goal of extending both the ability to live beyond 120 years and the duration of time spent living a healthy life is transitioning from a long-sought aspiration or recurring theme of science fiction into the defined mission of leading research centers. That includes efforts to narrow the gap between the global lifespan and healthspan.
“When we are in aging societies, we want to compress this gap as much as we can. That is one of the missions that science should take on in an interdisciplinary way,” said Nicole Wenderoth, a professor for neural control of movement and director of the Institute for Human Movement Sciences and Sport at ETH Zurich, who also is founding director of the Future Health Technology program at the Singapore-ETH Center.
The gap is largely caused by chronic diseases such as cancers, diabetes, and cardiovascular disorders, and “the idea is that rather than targeting each of these diseases on its own, we might target aging itself,” said Wenderoth, who contributed to the Radar and GESDA Summit. “And when we slow down aging, we might also just delay the time of getting some of these chronic diseases.”
She also believes more studies are needed to investigate connections between sleep and longevity, since, as she and other experts note in the Radar, sleep is the body’s natural process for restoration and repair and “sleep augmentation could be one vital tool in closing the gap between lifespan and healthspan.”
From Engine Repair to Full System Upgrade
The foundational work on epigenetic clocks, the molecular tools that measure a person’s biological age based on DNA methylation patterns, is moving into widespread clinical integration. While a pioneering study by researchers at the University of Zurich, published this year, validated the use of accessible interventions like Omega-3 fatty acids to measurably slow the clock by months, the immediate focus is shifting toward next-generation pharmaceutical agents.
The Radar presents geroscience as a field shifting from seeing aging as inevitable to one that is starting to believe it can be modulated to separate chronological years from frailty and age‑related disease risk. Researchers now deploy small molecules and proprietary compounds that directly target the hallmarks of aging, such as clearing senescent ‘zombie’ cells or boosting mitochondrial function, with the aim of potentially reversing our biological age by years, not months.
That biological biomarker, identified by the Radar as a key opportunity, enables objective measurement of health interventions and directly challenges traditional structures of society. As a result, scientists say we are moving toward a future where “chronological age” is less relevant than an individual’s independently verifiable “biological age,” leading to a society where elderly people are able to contribute as workers, investors, teachers, and mentors for decades longer.
Driving this clinical acceleration is the foundational science emerging from institutions like École Polytechnique Fédérale de Lausanne (EPFL), a cornerstone of Switzerland’s “Longevity Valley.” EPFL researchers, notably through the work on mitochondrial metabolism, identified pathways governing cellular energy and resilience. That directly informs the strategy of the new Center for Experimental and Translational Longevity Research (CERL) at EPFL Innovation Park.
CERL is actively working on translating these mitochondrial boosters and cellular repair compounds into human therapeutic treatments, aiming to provide a comprehensive “system upgrade” to the aging body rather than simply treating individual diseases. The EPFL-driven initiative is consolidated under the umbrella of the Swiss Healthy Longevity Campus, positioning Switzerland as a global leader in integrating these diagnostics and therapeutics.
The work builds on Switzerland’s historic strength in health and personalized medicine, leveraging its deep experience in pioneering technologies, top-tier health care, and a regulatory environment focused on scientific innovation.
Cancer Enters the Chronic Era
As people live longer and scientists increasingly focus on efforts to lengthen our healthspan, age-related illnesses are on the rise. Yet, predictions that cancer would transition into a chronic, manageable disease have been proving remarkably accurate, largely thanks to recent breakthroughs in gene and nucleic acid therapies.
This foundational work is visible at GESDA’s home base: EPFL researcher Andrea Ablasser, who recently won the prestigious Paul Marks Prize for Cancer Research from Memorial Sloan Kettering Cancer Center, has advanced this transition. Her work on the cGAS-STING signalling pathway, a central alarm for cellular stress, has been critical in developing targeted medicines that strengthen the immune defense against tumors.
Following the initial success of mRNA vaccines during the pandemic, companies like BioNTech have accelerated their personalized cancer immunotherapies. By the end of 2025, many of these tailored neoantigen vaccines are moving aggressively into or completing pivotal Phase II trials, with major Phase III studies anticipated in the coming year.
The technology, which programs the body’s own immune system to identify and destroy cancer cells with unprecedented precision, represents a major leap toward reprogramming the body’s immune system to provide a durable, long-term defense. Meantime, the democratization of gene technology continues unabated. The cost of sequencing a human genome has continued to plummet, making advanced genetic editing and somatic cell engineering — the treatment of conditions caused by the malfunction of several genes including cancer, diabetes, and cardiovascular diseases — more economically viable for national health systems.
The goal is no longer just cure, but intervention and prevention at the cellular level, helping to compress the time lost to age-related illness, a sentiment echoed by researchers contributing to the Radar.
The Dawn of Two-Tiered Aging
As scientific progress accelerates, the ethical challenge of the equity debate identified by the Radar has become the most pressing governance crisis. In a world of both sensationalized hype and fear-based dystopias surrounding radical health, the Radar offers a sober reality check: The emergence of a genuine biological age gap could fracture society.
High-end longevity interventions, personalized gene therapies, and access to state-of-the-art epigenetic diagnostics are initially centralized in wealthy nations and high-priced clinics in places like Switzerland. The result is the prospect of a two-tiered aging process: one cohort benefiting from a biologically decelerated, 100+ year healthspan, and another cohort subjected to the traditional diseases and disabilities of old age.
Given the immense costs of novel gene and immune therapies, the United Nations and other international bodies are leading global efforts to prioritize regulatory frameworks that mandate accessibility and affordability. Misinformation and deeply rooted concerns about social inequality, coupled with fears of losing mental autonomy through neurotechnology, are increasingly seen as policy questions to be addressed by researchers, policymakers, and diplomats globally.
The challenge, as illustrated in the Radar, is clear for anticipatory science and diplomacy: the radical health extension opportunity must benefit all populations, rather than becoming the ultimate source of deepening human division, as scientists work to narrow the gap between the global lifespan and healthspan.
“The mission for science,” said Wenderoth, “is whether we can actually change this trajectory.”
Where the science and diplomacy can lead us
The 2026 GESDA Science Breakthrough Radar®, distilling the insights of 2,390 leading researchers from 89 countries, shows the time for debate is over. The question for diplomats and political leaders is no longer if longevity science will cross the clinical threshold, but how they will urgently design policy and regulatory frameworks to ensure this new human potential does not become the ultimate source of deepening human division.
Key Radar references:
→Augmenting sleep to slow down biological aging — Until recently, medical science treated aging as an inevitable and largely untreatable process, but this view is increasingly being challenged. The field of longevity science is uncovering the underlying mechanisms that drive aging and developing interventions that can slow down or even partially reverse them. Sleep augmentation could be one vital tool in closing the gap between lifespan and healthspan.
→2.2 Cell and gene engineering — Gene editing has achieved significant successes in a range of areas and has gained regulatory approval for targeting cancer, eye diseases and blood diseases. Its next act will be to move from rare and difficult diseases into more common disorders and cancers, and from there into cracking the fundamentals of aging.
→2.3. Healthspan extension — Aging was once thought to be an inescapable fact of life. Many decades after scientific insights significantly extended human lifespan, research is making inroads into improving our healthspan, the amount of time we lead a healthy, productive existence. Certain parts of the dark genome seem to be involved in some aging processes, and finding ways to intervene could prove impactful. Investigations of lifestyle modification are also proving fruitful.
→2.3.1 Biomarkers of aging — Some people age better than others due to the interplay of their genes with environmental factors. As a result, the number of years that a person lives is an unreliable predictor of future health and longevity. A person’s “biological age,” based on an analysis of factors known as biomarkers, is more useful. Research has identified a vast range of biomarkers to help chart the accumulation of molecular and cellular damage that leads to age-dependent deterioration.
→2.3.2 Rejuvenating the epigenome — The aging process is associated with changes in the mechanisms that regulate gene expression, including DNA methylation, histone modification and chromatin remodeling. Though the underlying DNA sequence remains unchanged, these disruptions to the epigenome impact cell and tissue resilience against disease. Resetting an aged epigenome to a youthful state therefore offers the prospect of rejuvenation rather than merely slowing the pace of aging: it makes cells, tissues and organisms actually younger rather than simply slowing their decline.
→2.3.4 Lifestyle modification — Strong epidemiological evidence confirms environment and behavior are important for longevity. Sleep, nutrition, exercise and other healthspan-affecting choices can cause healthy and unhealthy aging to diverge.
→2.5.1 Cellular (re-)programming and tissue development — Through a deeper understanding of structures and processes in living cells, scientists are learning how to reprogram human cells. Cellular reprogramming can be used to reverse age-related changes in cells, enabling study of mechanisms of human aging in vitro. It may also shed light on developmental disorders.
→5.4 Fungal biology — Fungi are involved with nearly every organism and ecosystem on Earth. They have changed the course of human civilization: their yeast makes our bread and beer, their self-defense chemicals underpin important antibiotics and now their enzymes promise breakthroughs from greener biofuels and wastewater treatment to potential anti-aging drugs.











